The battery is the car's electrical heart. It stores the power that starts the engine and supplies all the car's electrical needs when the generating system cannot cope. It is also one of those components that requires little maintenance. Checking the electrolyte level is usually all that is necessary. Modern developments are relieving the car owner of even that responsibility.
All car batteries are of the lead-acid type, which means that they store electricity by harnessing the chemical effect that certain types of acid have upon lead. A battery is generally made up of a number of cells, which are linked together. Each cell gives an average output of two volts, so the usual 12-volt battery has six cells and the six-volt battery has three cells. The majority of batteries have a line of filler caps along the top and there is usually one cap for each cell.
The internal construction of each cell is identical; there are two sets of vertical plates, one positive, the other negative. The plates are metal grids normally made from an alloy of lead. In a fully charged battery there is a coating of lead peroxide on the positive plates and one of "spongy" lead on the negative plates. Each cell is filled to a level just above the top of the plates with a solution of sulphuric acid, called the electrolyte. The electrolyte contains a high proportion of distilled water. There is also an insulating plate fitted in the cells, between the positive and negative plates. Its purpose is to keep them from touching.
All the positive plates in a cell are connected together at the top, as are all the negative plates. The cells themselves are interconnected, positive to negative. The external connections to the car's electrical system are made at terminals on top of the battery, the positive terminal at one end and the negative at the other. Car electrics are conventionally of the "earth-return" type , so one of the main terminals, normally the negative, is attached directly to the bodywork through a short earthing strap.
The capacity or power of a battery depends on the total area of the lead plates and the size chosen for a particular car is a compromise between the likely electrical demand and the considerable bulk and cost of the component. Capacity is measured in ampere-hours (Ah) which is the current available multiplied by the number of hours for which it will flow. A common battery has a capacity of 40Ah, which means that it can supply a current of 4amps for ten hours. A 50Ah battery could supply 5amps over the same period.
How A Battery Works
When electricity is drawn from a charged battery, the current flow in the battery causes electrolysis of the electrolyte to take place in each cell. Electrolysis of the water in the electrolyte is a form of decomposition and produces oxygen ions and hydrogen ions, which are carriers of electric charges. The oxygen ions are negatively charged, while the hydrogen ones are positively charged. These charges cause the oxygen ions to seek the positive plates and the hydrogen ions to seek the negative plates. In the same way, the sulphuric acid itself is decomposed into hydrogen ions.
When these various ions reach the electrodes to which they are attracted, they give up their electric charges and react with the active material on the plates so causing current to leave the battery. This tends to convert both plates into lead sulphate, while the sulphuric acid rends to become water. The electrolyte is consequently diluted and its specific gravity falls. This chemical reaction would be completed only if the battery were fully discharged, as can happen should a car be left parked with its lights on for a long time.
Unlike A Phone, You Shouldn't Fully Discharge A Car Battery
In practice, full discharging should be avoided on two counts. First, since lead sulphate is very bulky, heavy sulphation of the plates could cause the grids to crack and some of the coating to fall off. In addition, recharging may be ineffective if a battery is left fully discharged, since the lead sulphate becomes increasingly difficult to convert back to its original materials. The voltage of a cell falls quickly from its maximum figure of 2.2 volts to a little over 2 volts once discharge begins. It remains almost constant at that value until the battery is nearly fully discharged, at which stage the voltage reduction is rapid and sulpnation becomes considerable, unless a charging current is quickly applied.
Once discharged or "flat", a battery can be recharged by passing a current through it. This is done automatically on the car by the dynamo or alternator, but if the battery becomes completely discharged it may be necessary to recharge it from the mains, a job which the DIY mechanic may often find himself doing during the winter. During charging, the current flows through the battery in the other direction-in at the positive terminal instead of out from it. The charging process is therefore the reverse of the discharging one; the positive plates revert to lead peroxide and the negative plates to lead. Some of the water is also converted back into sulphuric acid.
The charging equipment of modern cars is so designed that it provides a relatively high charging current when the battery most needs it. As the battery approaches full charge, though, and its voltage rises, the current is i educed, by the voltage regulator, to a low level. This not only ensures the full reconversion of the active material, but minimizes the gassing that occurs at the plates when the battery is fully charged. Violent gassing, which looks as though the electrolyte is boiling, can cause active material to be dislodged from the plates. Chemical activity and therefore the power a battery can deliver is related to the temperature. A battery is less effective for starting purposes in winter than in summer, when the greater viscosity of the cold oil in the engine further increases the power required to turn it. This often explains the reluctance of some cars' engines to start in really cold weather.
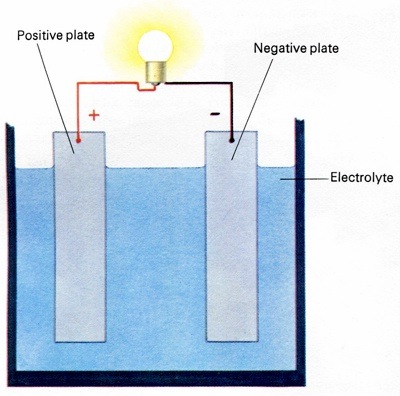 Each battery cell has two sets of plates which are surrounded by an acid solution called the electrolyte. When electricity is drawn from a charged cell, the current flow causes a chemical reaction to occur.
Each battery cell has two sets of plates which are surrounded by an acid solution called the electrolyte. When electricity is drawn from a charged cell, the current flow causes a chemical reaction to occur.
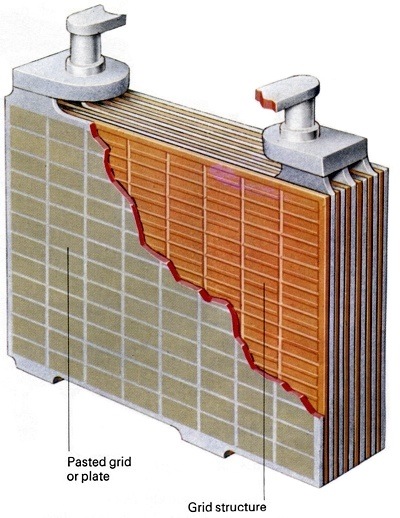
A cutaway image of the car battery cell, showing insulating plates fitted to keep the negative and positive plates from touching.
|
The State Of Charge Of A Car's Battery
The state of charge of a car's battery can be assessed by measuring the specific gravity of the electrolyte. Specific gravity (SG) is the weight of a substance compared with the weight of an equal volume of water. The SG of water is taken to be 1.00. Assuming the cells have been correctly filled in the first place, the SG of the electrolyte in a fully charged battery is about 1.28 (that is, 28 per cent heavier than water), but the figure drops to 1.11 to 1.13 (only 11-13 per cent heavier than water) at full discharge, because of the extra water in the electrolyte. The SG of the acid can be quickly assessed by a hydrometer.
This simple device consists of a small float in a glass tube marked with a scale. It has a nozzle at one end and a rubber bulb at the other. The electrolyte from each cell in turn is drawn into the tube by squeezing and releasing the bulb, whereupon the SG is simply and easily read off" on the scale. Since water evaporates quickly from the electrolyte of a conventional battery in service, but the sulphuric acid does not, a fall in liquid level is accompanied by a rise in the SG. This is why topping-up the cells with pure water is necessary from time to time, to restore the level of electrolyte and the SG. Screw-in plugs or special lids are provided in the top of a normal battery to enable the topping-up to be done. Some car owners use ordinary tap water for topping-up, but this is not recommended by the battery manufacturers because such water can contain chemicals that have an adverse effect on the life of the plates.
The life of a battery varies considerably according to the operating conditions and the regularity of topping-up, but typically it is in the region of two to three years. Old age manifests itself in the battery becoming increasingly unable to take a charge, or to hold it, when not in use. Sulphating of the plates is the cause of the battery being unable to take a charge and it arises from leaving the battery too long in the discharged condition. If the cells will not hold a charge, on the other hand, this is due to the plates (and sometimes the separators) having been damaged through vibration or unduly rapid charging or discharging.
Improving the Battery
Many improvements have been made to the lead-acid battery since it was first used in cars. Ways have been found to make the plates and their mountings stronger, while the separators are also considerably better than they were. Porous rubber and plastic materials are now used and these are much stronger than the wood and other substances previously employed. They have enabled the separator thickness to be reduced so that the battery can be made more compact. The weight of the modern battery has also been cut and, conversely, its strength increased through the rapid development and use of moulded plastic cases.
A more fundamental advance has been accomplished during the past few years, leading to the "maintenance-free" (or MF) battery. This type of battery is widely fitted in the USA, where it was invented, and has been standardized for General Motors' entire range of cars. Other American makers, notably Ford, intend to follow suit. European battery firms have been slower to produce MF batteries but their interest was fanned by GM's decision in 1978 to start exporting its own Delco range to its companies in Europe. The maintenance-free battery is just what the name says: it does not require topping-up at any point in its life and is not even equipped with any holes for the purpose, being filled and sealed in the factory.
In America it has cost perhaps a fifth as much again as a good-quality conventional battery. In return it claims to eliminate the possibility of premature battery failure through neglect by the lazy or forgetful owner. Apart from the elimination of topping-up, the MF battery greatly reduces three other deficiences of the earlier design-poor shelf life (the time a battery can be stored before sale), deterioration in service and corrosion of the terminals. All these disadvantages of the orthodox battery stem in some way from one feature of its construction. For many years the metal antimony has been added to the lead from which the plate grids are made, to give them adequate strength. Unfortunately, however, the antimony also acts as a sort of slow poison, creeping through the electrolyte from positive plates to negative.
This causes deterioration of the battery, even when it is new and standing on a shelf, which limits the time that unused batteries can be stored. The fact that brand-new batteries could deteriorate in this way forced manufacturers to send out "dry" batteries from the factory, the acid being added just before the battery was put into service. In overcoming one problem, however, this arrangement introduced others. The most important was the difficulty of ensuring that the battery would be up to standard after filling. However carefully it was checked beforehand, there was always the possibility that charging would not be satisfactorily carried out by the retailer.
Because of the poisoning effect of antimony, the amount used in batteries was reduced by about a third during the 1950s. Although this reduction significantly slowed the rate of deterioration, Delco and Gould Inc in the United States felt that much more remained to be done. Both carried out an extensive research programme during the 1960s, and both came up with the same solution - the elimination of antimony by the use of calcium-lead alloys for the plate grids. Calcium-lead was by no means a new material in battery manufacture, but it had previously been used only for the few thick plates of telephone batteries, not the many thin ones necessary for a vehicle battery. Nevertheless, ways were found of adapting battery design and manufacture so that this metal could be used.
During the research, the American firms found another bonus. Not only did the use of calcium-lead extend the shelf life of a battery by at least five times, but it also greatly diminished the amount of gassing that occurred when full charge was reached. The rate with which water was lost from the electrolyte became very low and terminal corrosion - the result of acidic vapour being blown out of the cell vents by the gassing - was almost non-existent. What started as a search for longer shelf life and more consistent performance thus developed into a project of benefit to the car owner and pointed towards the development of batteries that would need no servicing during their working life. Realizing this ideal meant making other changes and these were investigated and perfected, until both Delco and Gould reached the stage of "field trials" with the vehicle manufacturers in the early 1970s.
To ensure that no topping-up was necessary, the first essential was to provide a high electrolyte level over the plates. To achieve this without either reducing the depth of the plates or deepening the case, the plates had to be seated on the bottom of the cells. Formerly they were slightly raised to leave a space in which any detached active material could collect. It followed that the positive plates, which are affected most by detached material, had to be enclosed to prevent any such material from building up at the bottom of the container and causing a short-circuit.
Incorporated in the lid of the battery are combined gas traps and condensers. These further reduce the rate of water loss and are inserted after the battery has been filled with electrolyte at the factory. Although some premature failures of MF batteries have occurred in the United States, where other makers have now joined Delco and Gould in their production, the frequency of such troubles appears to be lower than for the conventional type. There is no doubt, though, that these relative newcomers do live up to their description in that none of the failures has resulted from shortage of electrolyte or terminal corrosion, which are the usual consequences of lack of maintenance. The MF battery has other benefits too. Because access to the battery is required only for its initial fitting and replacement, it no longer has to be installed under the bonnet, where space is often at a premium and the environment far from ideal. It can now be relegated to a part of the vehicle where it is less obtrusive.
Maintenance Free versus Low-Maintenance Car Battery
While Gould and Delco were developing their calcium-lead batteries, other manufacturers in the United States and Germany adopted a different approach. They felt that an acceptable increase in shelf life and reduction in maintenance could be achieved through a further lowering of the antimony content of the plate grids. By using additional alloying elements, the companies managed to get the antimony down to 2\ to 3 per cent, and low-antimony batteries of this type are in large-scale production in the two countries. In the United States, of course, they are in direct competition with the MF variety, and being usually 10 per cent cheaper, they have a considerable advantage. These batteries are also sometimes referred to as "maintenance-free" but, since topping-up plugs are necessary, "low-maintenance" is generally regarded as a more accurate description.
Low-maintenance batteries are certainly superior to the normal higher-antimony ones, but their advantage in all the major aspects of battery performance is much less than in the MF design. For example, the shelf life, although about half as long again as that of the ordinary battery, is still less than a third of the MF's life.Another bonus provided by the MF battery is its greater resistance to overcharging, as could occur if a car's voltage regulating equipment had been wrongly set. Where as the low-antimony battery is around 25 to 30 per cent better than the normal one, the MF unit shows at least three times as much resistance to overcharging from this cause.
Cutting out battery maintenance is very much in line with modern "sealed for life" trends in other aspects of contemporary car development such as some gearboxes and transmission components. Because of these undoubted advantages, it would seem likely that the MF battery has a wide potential market in Europe as well as in the United States. The principal worry for European manufacturers is whether motorists will pay the higher price for the better component. The public may not demand MF batteries until the price becomes more competitive and the price cannot be brought down until production is high, which, in turn, cannot occur until the public demand is strong and steady and sales are high.
Whatever the commercial complexities, the fact remains that the maintenance-free battery is a milestone in vehicle electrics. In comparison, the low-antimony type can be judged as merely an evolutionary improvement. Even so, it became a standard low-cost alternative to the MF battery all over the world.



